Regulatory Pharmacist
Lifestrong Marketing Inc.
Job Description
Benefits
Government Mandated Benefits
13th Month Pay, Pag-Ibig Fund, Philhealth, SSS/GSIS
Others
Company Social Events
Perks Benefits
Employee Discount
Time Off & Leave
Bereavement Leave, Birthday Leave, Maternity & Paternity Leave, Sick Leave, Vacation Leave
- Prepares and check submission of License to Operate renewal and other inter-related regulatory correspondence with FDA.
- Timely monitoring of product, license, permit and other licenses for renewal and ensure that all FDA license, certificates, and permits are updated and valid.
- Reviews Standard Operating Procedure based on the actual activity.
- Ensures that all pertinent documents for Cosmetic, Food, Drug and Medical Device are available for FDA inspection.
- Compiles and maintain complete technical data of each product by timely update of LTO’s master file.
- Ensures reliable IPO/Trademark monitoring
- Assists in addressing all customer inquiries by providing retention and product test results within 2 working days upon request.
- To ensure that product samples of new batches are distributed for testing 7 working days upon receipt.
- To provide product testing to Purchasing Dept. 20 working days after product test distribution or before product release to the market (for urgent testing).
- Ensures that formulation conforms to FDA standards and is within the approved limit.
- Ensures that labels of new and existing items conform to FDA Standards.
- Checking of e-Portal for newly approved CPN/CPR’s. Download, file and update the Notifications Summary.
- Generates barcode and monitoring of registration on-line.
- Ensures that Vidamed warehouses are complying with FDA requirements.
- Assists FDA inspector with the needs during inspection and makes sure that company is compliant.
- Conducts product tests for all finished goods distributed and imported by the company.
- Maintain current knowledge of relevant regulations in Cosmetic/Food/Medical device/Drugs and keeping up to date with changes in regulatory legislation and guidelines.
- Assists in updating related departments and regulatory head on latest regulatory requirements and FDA advisories.
- Coordinates the schedule of pest control.
- Monitoring in conducting annual audits of all active suppliers.
- Coordination with manufacturers for Product Information File for filing based on ASEAN Cosmetic Directive.
- Conducts a Product Information File for filing based on ASEAN Cosmetic Directive.
- Processing of initial and renewal of agreements and request for updated GMP and ISO of all active suppliers.
- Perform other related duties as required by FDA and other tasks assigned as needed.
Angel Joy Cauilan
Talent Acquisition Associate Lifestrong Marketing Inc.
Active within three days
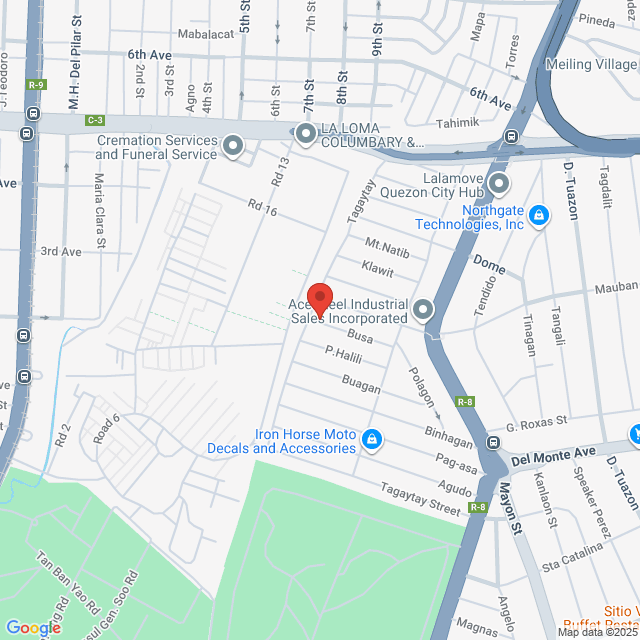
Working Location
98 Busa, Caloocan, 1404 Metro Manila, Philippines
Posted on 22 October 2025
Explore similar jobs
View more similar jobsRegulatory Pharmacist
 Main Source Manufacturing and Trading Corp.
Main Source Manufacturing and Trading Corp.₦492.8-615.9K[Monthly]
On-site - Caloocan1-3 Yrs ExpBachelorFull-time

MAIN SOURCE MANUFACTURING AND TRADING CORPHR Manager
Regulatory Affairs Pharmacist Manager - 5 yrs Experience
 Dempsey Resource Management Inc.
Dempsey Resource Management Inc.₦985.5K-1.1M[Monthly]
On-site - Caloocan5-10 Yrs ExpBachelorFull-time
Aisa Refugia
Regulatory Affairs Pharmacist
 Allegens Healthcare Philippines Inc.
Allegens Healthcare Philippines Inc.Negotiable
On-site - Makati1-3 Yrs ExpBachelorFull-time

Nguyen Tuyet NuHR Manager
Regulatory Pharmacist
 Ambica International Corporation
Ambica International Corporation₦492.8-739.1K[Monthly]
On-site - PasayNo Exp RequiredBachelorFull-time
Race SeldaRecruitment Supervisor
Regulatory Pharmacist
 Raya Regenerative and Preventive Medicine
Raya Regenerative and Preventive Medicine₦739.1-985.5K[Monthly]
On-site - Makati<1 Yr ExpBachelorFull-time

Justine GamboaHR Associate
Sign In to Chat with Boss
Bossjob Safety Reminder
If the position requires you to work overseas, please be vigilant and beware of fraud.
If you encounter an employer who has the following actions during your job search, please report it immediately
- withholds your ID,
- requires you to provide a guarantee or collects property,
- forces you to invest or raise funds,
- collects illicit benefits,
- or other illegal situations.

